Abstract
Determination and dissipation kinetics of pymetrozine and spirotetramat in green bean were studied using a QuEChERS method coupled to high-performance liquid chromatography-tandem mass spectrometry. Pymetrozine recoveries ranged between 88.4–93.7%, with relative standard deviation (RSD) of 5.5–14.4%. For spirotetramat the recoveries ranged between 91.7–103.4%, and the RSD were in the range of 3.2 to 12.4%. The limits of quantification (LOQs) were 0.01 mg/kg and 0.005 mg/kg for pymetrozine and spirotetramat, respectively.
The developed analytical method was used to study the degradation rates of pymetrozine and spirotetramat in green bean grown in open field. Results showed that pymetrozine and spirotetramat followed the first-order kinetics model with half-lives of 3.3 days and 4.2 days, respectively. Furthermore, risk assessment was carried out which showed that, the chronic risk quotient (RQc) values for pymetrozine and spirotetramat were much lower than 100%. The present results indicated that the health risks posed for consumers by the pymetrozine and spirotetramat residues were negligible at the recommended dosages.
Similar content being viewed by others
Introduction
Green bean (Phaseolus vulgaris L.) is a common food worldwide which is eaten either raw or cooked. It is one of the major leguminous crops grown in Egypt, the green pods and dry seeds are marketed for local consumption and also for exportation. (Badawy et al. 2020). Egypt's annual production amounts to about 265 thousand tons (FAOSTAT 2020). With such high productivity rate, Egypt ranks tenth among the green bean exporting countries in the world. In addition to the economic value of green beans, it improves the soil quality as the parts of roots remaining in soil after harvesting is considered valuable fertilizers enriching the soil (Fahad et al. 2015a).
The green bean plant is susceptible to a score of insect pests, including aphids, thrips, leaf worms and others that affect both the quality and quantity of the yield, which requires regular insecticides applications. In Egypt, the Agricultural Pesticides Committee (APC) recommends the use of different classes of pesticides on this crop to control various pests and diseases (Agricultural Pesticides Committee 2020). Spirotetramat and pymetrozine pesticides are widely used to control sucking pests in green beans. Even though, these pesticides are beneficial in protecting green beans from pests, they may accumulate in the edible parts causing potential health risk to consumers (Ahmed et al. 2014; Xu et al. 2021).
Spirotetramat, cis-3-(2,5-dimethlyphenyl)-8-methoxy-2-oxo-1-azaspiro (Fig. 1a), belongs to the chemical class of tetramic acid derivative. It is one of the recommended and effective insecticides used to control widespread insects such as aphids and thrips in vegetables and fruits by inhibiting the action of acetyl-CoA carboxylase (Brück et al. 2009; Kay and Herron 2010; Kumar et al. 2009; Kumar and Kuttalam 2009; Smiley et al. 2011).
Pymetrozine, 4,5-dihydro-6-methyl-4-[(3- pyridyl methylene)-amino]-1,2,4-triazin-3(2H)-one (Fig. 1b) is another effective pesticides against sucking insects. It is a pyridine azomethine-based insecticides, that inhibits nerve-muscle interaction of the sucking insects (Lashkari et al. 2007; Li et al. 2011; Shen et al. 2009). The Environmental Protection Agency (EPA) has deemed pymetrozine a "possible" human carcinogen (Zhang et al. 2007). Therefore, it is necessary to monitor the residues of pymetrozine in green beans.
The irrational use of pesticides raised concerns due to their risk to human health. In this context, governments and international organizations are committed to regulate and control the use of pesticides to protect consumers’ health. Setting a maximum acceptable residue limit (MRL) is an integral element of Good Agricultural Practices (Handford et al. 2015). Moreover, a pre-harvest interval period (PHI) has been also introduced as another precautionary measure to protect consumers (MacLachlan and Hamilton 2010).
Several studies were conducted to monitor residue of spirotetramat or pymetrozine in many crops including cotton (Pandiselvi et al. 2010), grapes (Mohapatra et al. 2015; Vemuri et al. 2014), chilli (Chahil et al. 2015), mangoes (Mohapatra et al. 2012b), spinach (Chen et al. 2016), tomatoes (Abd-Alrahman and Kotb 2020; Abd Al-Rahman et al. 2012), strawberry (Xu et al. 2021) and many other fruit and vegetable crops (Han et al. 2013; Jia et al. 2019; Li et al. 2011; Singh et al. 2013). However, to the best of our knowledge no data has been reported about dissipation of spirotetramat and pymetrozine in green beans under field conditions.
To monitor spirotetramat and pymetrozine residues in food commodities different analytical methods were used such as, high performance liquid chromatography (HPLC) (Abd-Alrahman and Kotb 2020; Abd Al-Rahman et al. 2012; Cabizza et al. 2007; Chahil et al. 2015; Hong et al. 2011; Mohapatra et al. 2012a; Pandiselvi et al. 2010; Shen et al. 2009; Singh et al. 2013; Vemuri et al. 2014), ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS/MS), liquid chromatography with tandem mass spectrometry (LC–MS/MS) (Chen et al. 2016; Dias et al. 2013; Fernandes et al. 2014; Jia et al. 2019; Li et al. 2011, 2016; Xu et al. 2021; Zhang et al. 2015; Zhu et al. 2013) and gas chromatography mass spectrometry (GC/MS) (Jang et al. 2014; Mohapatra et al. 2015). Considering the extensive use of spirotetramat and pymetrozine in green beans there is an urgent need to develop a quick, precise and accurate method to simultaneously determine them.
This study aimed to develop a simple, accurate, and rapid method for simultaneous quantitation of spirotetramat and pymetrozine residues in green beans using UPLC–MS/MS. A continuous application approach using single and double recommended doses was carried out to investigate the dissipation patterns, residue levels, and risk assessment of spirotetramat and pymetrozine in green beans cultivated in open field conditions at different time intervals. The results could provide guidance for the safe use of spirotetramat and pymetrozine in green beans under open field conditions.
Materials and methods
Chemicals and reagents
Certified standard samples of pymetrozine (purity, 99.4%) and spirotetramat (purity, 99.4%) were purchased from ChemService (West Chester, PA, USA). HPLC grade acetonitrile (ACN) and methanol (MeOH), LC–MS grade formic acid, were obtained from Fisher Scientific (Loughborough, UK). Sodium chloride and magnesium sulfate anhydrous were supplied from Chem-Lab NV (Zedelgem, Belgium). Primary secondary amine (PSA, 40–60 μm) was obtained from Agilent Technologies (DE, USA). The formulations of pymetrozine (50%, wettable granule, WG), and spirotetramat (10 %, suspension concentrate, SC) were provided from the local markets.
Preparation of standard solutions
Standard stock solutions of pymetrozine and spirotetramat (each at 100 mg/L) were prepared by dissolving 10.06 mg of each standard in 100 mL ACN. The intermediate mixture standard solution of 10 mg/L was prepared in ACN by further dilution. Mixed standard working solutions with equal concentrations were serially diluted using ACN to construct calibration curves. All the solutions were stored at 4 °C.
Field trail
The field trials were carried out, during the growing season 2020, in Giza governorate, located south of Cairo. Green beans were cultivated in February 2020. Two independent experiments were carried out, one for each insecticide, with different plots (50 m2) for each insecticide. A buffer zone (15 m2) was made to separate adjacent plots to avoid cross-contamination.
Samples were collected 0, 2 h, 1, 3, 7, 10, 14 days post treatment to monitor each insecticide residues in green beans. Samples were immediately transferred to the laboratory, cut into small pieces about 3 cm and then frozen at -20 °C overnight, homogenized the next day using a Hobart food cutter (Hobart Corp., Troy, OH, USA).
Terminal residues
Pymetrozine and spirotetramat were applied 2 or 3 times each, at two dosage level 100 g a.i/ha (low level) and 200 g a.i/ha (high level) after the edible part of the fruits was formed. Representative samples were collected according to Codex guidelines (FAO and WHO 2019) at several pre-harvest Intervals (PHI). For the assessment of terminal residues, samples were taken at 3, 7 and 14 days after the last treatment.
Sample extraction
Quick, easy, cheap, effective, rugged, and safe (QuEChERS) is the most common technique used due to their simplicity, good purification efficiency, and low organic solvent consumption (AOAC 1990; Duan et al. 2018; Hong et al. 2011; Lehotay et al. 2005; Lehotay2007;Singh et al. 2013; Yang et al. 2015). The QuEChERS method includes an extraction step with acetonitrile (ACN) and partitioning using MgSO4. The extraction is cleaned up by primary secondary amine (PSA), octadecyl modified silica (C18), and graphite carbon black (GCB) dispersive solid phase extraction (Anastassiades et al. 2003b, 2003a).
The frozen homogenized green bean (10 g) was weighed into a 50 mL polypropylene centrifuge tube, and 10 mL ACN was added to it. Samples were extracted by vortex for 2 min after adding a piece of ceramic homogenizer in the tube. 1 g of sodium chloride and 4 g of anhydrous magnesium sulfate were added. The sample was hand-shaken again for 30 s. After centrifugation at 5000 rpm for 5 min, 0.2 mL of the top layer ACN was 5x diluted using ACN, then vortexed for 30 s. Finally, the tubes were filtered through a 0.22 µm nylon syringe filter for LC–MS/MS analysis.
LC–MS/MS
A Dionex Ultimate™ 3000 RS UHPLC+ focused system separation module Liquid Chromatograph (LC) system (Thermo Fisher Scientific, Austin, TX, USA) in combination with TSQ Altis triple quadrupole mass spectrometer (MS/MS) was used to perform the LC–MS/MS analysis. The chromatographic separation was performed on the Accucore RP-MS C18 column (100 × 2.1 mm, 2.5 µm film thickness; Thermo Scientific, Lithuania) at 40 °C, with an injection volume of 1 µL. The mobile phase consisted of water/acetonitrile (30/70. v/v) with 0.1% formic acid at a total run time of 7 min. The pesticide detection was performed using the multiple reaction monitoring (MRM) mode. The optimal MRM transitions, collision energies (CE), and radio frequencies (RF) of S-lens were optimized using a standard solution of 0.5 mg/L in 50/50 MeOH/H2O with 0.1% formic acid at a constant flow rate of 0.3 mL/min and injection volume of 5 µL in an infusion mode. The electrospray ionization was operated in a positive mode (ESI+). The capillary ion spray voltage was 3800 V, the ion source temperature was 325 °C. The sheath and Aux gas pressure were 40 and 10 Arb, respectively. Trace Finder software (version 4.1) packages were applied to acquire and process the data obtained. Under these conditions, the retention times of pymetrozine and spirotetramat were 0.71 and 1.89 min, respectively. The specific MS/MS parameters are given in Table 1 and Fig. 2.
Method validation
Method performance was validated in terms of linearity, specificity, matrix effect (ME), accuracy and precision, and limit of quantification (LOQ), according to SANTE/12682/2019 guidelines (SANTE 2021). Linearity was assessed through the coefficient of determination (R2), residuals, and factors (RF) derived from the constructed external calibration curves. The constructed calibration curves that prepared in the blank sample, and that prepared in acetonitrile, were used to determine the percentage of matrix effect (%ME) as follows:
where ME is the matrix effect, and SMMC and SSC are the slopes of the calibration curves in the matrix and pure solvent, respectively. A ME% of a positive value indicated that the matrix enhanced the analytical response, and a negative value showed that the matrix suppressed the analytical response. Blank green bean samples were analyzed to check the specificity of the method by observing if peaks occurred at or around the same retention time of the target analyte. The accuracy and precisions were estimated in recovery (%) and relative standard deviation (RSD, %), respectively. Blank green bean samples were spiked at four concentration levels of 0.005, 0.01, 0.1, and 1 mg/kg to confirm the method validity in the same day (intra-day repeatability) and three different days (inter-days repeatability). The LOQ was defined as the lowest spiked level achieving an acceptable recovery of 70–120% and precision of < 20%.
Calculations
Dissipation and terminal residue
The degradation rates of pymetrozine and spirotetramat was calculated using a first-order kinetic model illustrated by Eq. (2)
where Ct (mg/kg) is the residual levels of pymetrozine or spirotetramat at time t (days), C0 (mg/ kg) is the initial deposits. K is the first-order rate constant (day−1) obtained from the C0/Ct and t curve by regression analysis. The half-life (T1/2) is the time taken for a certain amount of pesticide to be reduced by 50%. The T1/2 was calculated by Hoskins’ formula (Eq. 3) (Hoskins 1961; Liang et al. 2011). The safe pre-harvest interval (PHI) was computed using Eq. 4.
Dietary intake risk assessment
The risks that may occur as a result of long-term dietary intake of the contaminated green beans with pymetrozine or spirotetramat was assessed using Eq. 5 and Eq. 6 (Malhat and Abdallah 2019).
The NEDI (mg/kg.bw/day) is the national estimated daily intake of the tested pesticide based on the Egyptian dietary intake. STMRi (mg/kg) is the supervised trials median residue value obtained from the field trials. Where Fi represents the consumption of green beans by the general population, and bw (kg) is the average body weight of adults (60 kg).
The risk quotient (RQ) was determined by Eq. 6
The ADI is the acceptable daily intake of pymetrozine (0.03 mg/kg.bw/day) (EFSA 2012) and spirotetramat (0.05 mg/kg.bw/day) (EFSA 2017).
Results and discussion
Method performance
To validate the specificity of the developed method, a representative blank sample of green beans was analyzed in triplicates to confirm that no matrix interfering peaks appears at the retention time of the target pesticides. Figures 3 and 4 showed that no interfering peaks appeared at or around the retention time of pymetrozine and spirotetramat, indicating that the method is specific. The selectivity of the method was confirmed by the identical retention time of pymetrozine and spirotetramat in the solvent and the matrix samples. The chromatograms of pymetrozine and spirotetramat resolved well in the solvent, blank, and fortified samples (Figs. 3 and 4).
Matrix-matched calibration curves of pymetrozine and spirotetramat were plotted for eight concentrations (0.001, 0.002, 0.005, 0.01, 0.025, 0.05, 0.1, and 0.2 μg/g). The calibration curves were linear with a correlation coefficient of r > 0.998, and response factors of ˂ 20%. The regression equations of SMMC were used for analytes quantification. The matrix effects of green beans were obtained using Eq. 1. Results showed that matrix of the green bean samples caused the suppression of pymetrozine responses with an ME equals to16.2%, while enhancement of spirotetramat responses were observed with an ME of 6.75%. To account for this effect this study used matrix-matched standard solutions to obtain more precise data (Table 2). The ME in UPLC-MS/MS is a consequence of the competition between the analyte and the complex matrix of the sample during ionization process in the electrospray ion source (Taylor 2005).
The LOQs of pymetrozine and spirotetramat were 0.01 and 0.005 mg/kg, respectively (Table 2). The LOQ of pymetrozine and spirotetramat was 70 and 400 times lower than the MRL set by the European Union Commission (EFSA 2017, 2012).
Table 3 shows method precision and trueness expressed as repeatability (RSD%) and recovery (accuracy) (SANTE 2021). The efficiency of the extraction method was validated based on the recovery results (Table 3). The recovery study was developed at four concentration levels of 0.005, 0.01, 0.1, and 2 mg/kg, using six consecutive extractions for each spiked level. The mean recoveries ranged between 88.4% and 93.7% with RSDr less than 11.6% for pymetrozine, and ranged between 95.1% and 103.4% with RSDr less than 7.7% for spirotetramat. The inter-days recovery and RSDR (n = 18) for the tested concentration levels ranged from 88.9 to 92.4% with RSDR less than 14.4% for pymetrozine, and 91.7 to 97.1% with RSDR less than 12.4% for spirotetramat. Recoveries at the different concentration levels of the tested pesticides in green beans samples were satisfactory and within the SANTE recovery limits (70% ≤ Recovery ≤ 120%) and repeatability (≤ 20%) for the samples (SANTE 2021). This specified the accuracy and reproducibility of the developed method.
Dissipation
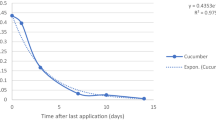
Figure 5 shows the dissipation rate of pymetrozine and spirotetramat in green bean after one application. The first-order kinetic equation described the degradation rates of pymetrozine and spirotetramat with correlation coefficients of 0.953 and 0.970, respectively (Fig. 5). The initial concentrations of pymetrozine and spirotetramat of 0.108 mg kg−1 and 0.513 mg kg−1, respectively, decreased gradually as time lapsed (Fig. 5). After 14 days 95% of pymetrozine and 90% of spirotetramat were degraded. The growth dilution could be one of the reasons behind dissipation, while precipitation is not likely to be an important factor due to the low water solubility of spirotetramat (30 mg/L, 20 °C) and pymetrozine (270 mg/L, 20 °C).
In this study, the half-live of pymetrozine was 3.3 days, which is lower than those reported by Zhang et al. (2015) of 2.3—2.6 days in rice straw, Xu et al. (2021) of 6.79–11.36 days in strawberry and Abd-Alrahman and Kotb (2020) of 1.31 days in tomato. Meanwhile, the half-live of spirotetramat in the present study was 4.2 days which is less than values reported in other studies on other crops where it ranged between 4.4–8.1 days in citrus (Zhang et al. 2015), 12.4 days in pear (Xun et al. 2019).
The lower half-lives reported in this study compared to the studies of Zhang et al. (2015), Xun et al. (2019), Abd-Alrahman and Kotb (2020) and Xu et al. (2021), could be due to difference in some environmental factors during the experiment such as temperature, humidity, salinity and light intensity. These abiotic factors have major effects on plants metabolic and catalytic activities which may affect the dissipation of pesticides (Fahad et al. 2017, 2015b, 2015a). Furthermore, the differences in growth rate and chemical constituents between the different crops could have an effect on the half-lives of pesticides (Saber et al. 2020).
Terminal residues
The terminal residues of pymetrozine and spirotetramat in green bean 3, 7 and 14 days after the last application are shown in Table 4. The residues of the two pesticides decreased by time whether were applied two or three times at the two tested concentrations (Table 4).
The concentrations of pymetrozine 3 and 7 days after application were higher when applied at double the recommended dose compared to applying the recommended dose. However, after 14 days the residues of pymetrozine decreased, reaching 0.081 ± 0.003 and 0.018 ± 0.007 mg kg−1 at double the recommended dose and the recommended dose, respectively. When spirotetramat was applied two and three times at recommended dose and twice the recommended dose based on PHI (14 days), residues of spirotetramat were 0.042–0.047 mg kg−1 and 0.043–0.047 mg kg−1, respectively. The European MRL for both pesticides, spirotetramat and pymetrozine in fresh beans with pods is 2 mg kg−1 (EFSA 2021, 2012). In the present study, the terminal residues at all studied time intervals and concentrations were far below the European MRL, which indicated good agricultural practice complying with consumer safety and product international trading (Saber et al. 2020).
Risk assessment
The potential risk to humans associated to the consumption of green beans with residues of pymetrozine and spirotetramat at the levels reported in this study was assessed using the RQ (Table 5). Results showed that the RQ values of pymetrozine and spirotetramat in green bean were all below 100% at the recommended dosage and also at twice recommended dosage applied two and three times. This indicated that the residue levels of pymetrozine and spirotetramat in green bean do not pose hazardous effects to consumers at the studied concentrations.
Conclusions
The present study has validated a QuEChERS extraction method for the residual analysis of pymetrozine and spirotetramat in green bean using LC–MS/MS. Field experiments were conducted to study the dissipation dynamics and terminal residues of pymetrozine and spirotetramat in green bean. In addition, a risk assessment study was conducted on the dietary intake of pymetrozine and spirotetramat in green bean based on their residues after field trials. In this study, the average recovery of pymetrozine and spirotetramat in green bean was ˃88.4%, with precision of ˂ 14.4% at fortification levels of 0.005, 0.01, 0.1 and 2 mg/kg. The LOQs of pymetrozine and spirotetramat were 0.005 mg/kg and 0.01 mg/kg, respectively. Field experiments showed that the degradation of pymetrozine and spirotetramat in green bean followed a first order reaction kinetic equation. Half-lives of pymetrozine and spirotetramat were 4.2 and 3.3 days respectively. The RQ values were far below 100%, indicating that pymetrozine and spirotetramat at the residue levels reported in the field experiments, do not pose risk to consumers. The present study has provided a scientific basis for the safe application of pymetrozine and spirotetramat in green bean under Egyptian field conditions.
Data availability
Not applicable.
References
Abd Al-Rahman SH, Almaz MM, Ahmed NS (2012) Dissipation of Fungicides, Insecticides, and Acaricide in Tomato Using HPLC-DAD and QuEChERS Methodology. Food Anal Methods 5:564–570. https://doi.org/10.1007/s12161-011-9279-0
Abd-Alrahman SH, Kotb GAM (2020) Dissipation kinetics of pymetrozine in tomato field ecosystem. Egypt J Hosp Med 81:2305–2309. https://doi.org/10.21608/ejhm.2020.131248
Agricultural Pesticides Committee (2020) Pesticides committee technical recommendations for agricultural pest control. Arab Republic of Egypt Ministry of Agriculture and Land Reclamation Egypt Agricultural Pesticides Committee 1–285. http://www.apc.gov.eg/Files/Releases/Recom.20-English.pdf
Ahmed MT, Greish S, Ismail SM, Mosleh Y, Loutfy NM, El Doussouki A (2014) Dietary Intake of Pesticides Based on Vegetable Consumption in Ismailia, Egypt: A Case Study. Hum Ecol Risk Assess 20. https://doi.org/10.1080/10807039.2013.775893
Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ (2003a) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for. J AOAC Int 86:412–431
Anastassiades M, Maštovská K, Lehotay SJ (2003b) Evaluation of analyte protectants to improve gas chromatographic analysis of pesticides. J Chromatogr A 1015:163–184. https://doi.org/10.1016/S0021-9673(03)01208-1
AOAC (1990) AOAC: official methods of analysis. Assoc Off Anal Chem 1:73–80
Badawy MEI, Mahmoud MS, Khattab MM (2020) Residues and dissipation kinetic of abamectin, chlorfenapyr and pyridaben acaricides in green beans (Phaseolus vulgaris L.) under field conditions using QuEChERS method and HPLC. J Environ Sci Heal - Part B Pestic Food Contam Agric Wastes 55:517–524. https://doi.org/10.1080/03601234.2020.1726701
Brück E, Elbert A, Fischer R, Krueger S, Kühnhold J, Klueken AM, Nauen R, Niebes JF, Reckmann U, Schnorbach HJ, Steffens R, van Waetermeulen X (2009) Movento®, an innovative ambimobile insecticide for sucking insect pest control in agriculture: Biological profile and field performance. Crop Prot 28:838–844. https://doi.org/10.1016/J.CROPRO.2009.06.015
Cabizza M, Satta M, Falconi S, Onano M, Uccheddu G (2007) Degradation of cyprodinil, fludioxonil, cyfluthrin and pymetrozine on lettuce after different application methods. J Environ Sci Heal Part B 42:761–766. https://doi.org/10.1080/03601230701549271
Chahil GS, Mandal K, Sahoo SK, Singh B (2015) Risk assessment of mixture formulation of spirotetramat and imidacloprid in chilli fruits. Environ Monit Assess 187:1–8. https://doi.org/10.1007/S10661-014-4105-Y/TABLES/4
Chen X, Meng Z, Zhang Y, Gu H, Ren Y, Lu C (2016) Degradation kinetics and pathways of spirotetramat in different parts of spinach plant and in the soil. Environ Sci Pollut Res 23:15053–15062. https://doi.org/10.1007/s11356-016-6665-6
Dias CM, Oliveira FA, Madureira FD, Silva G, Souza WR, Cardeal ZL (2013) Multi-residue method for the analysis of pesticides in Arabica coffee using liquid chromatography/tandem mass spectrometry 30: 1308-1315. https://doi.org/10.1080/19440049.2013.801088
Duan X, Tong L, Li D, Yu Z, Zhao Y (2018) a multiresidue method for simultaneous determination of 116 pesticides in Notoginseng Radix et Rhizoma using modified QuEChERS coupled with gas chromatography tandem mass spectrometry and census 180 batches of sample from Yunnan Province. Chromatographia 81:545–556. https://doi.org/10.1007/s10337-017-3460-6
EFSA (2021) amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for imidacloprid in or on certain products. Off J Eur Union 380:5–19
EFSA (2012) Reasoned opinion on the modification of the existing MRLs for pymetrozine in lamb’s lettuce and beans (with pods). EFSA J 10. https://doi.org/10.2903/j.efsa.2012.2939
EFSA (2017) Setting of maximum residue levels for spirotetramat in pomegranates and various vegetables. EFSA J 15. https://doi.org/10.2903/j.efsa.2017.4684
Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, Khan FA, Khan F, Chen Y, Wu C, Tabassum MA, Chun MX, Afzal M, Jan A, Jan MT, Huang J (2015a) Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ Sci Pollut Res 22:4907–4921. https://doi.org/10.1007/s11356-014-3754-2
Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, Hassan S, Shan D, Khan F, Ullah N, Faiq M, Khan MR, Tareen AK, Khan A, Ullah A, Ullah N, Huang J (2015b) Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul 75:391–404. https://doi.org/10.1007/s10725-014-0013-y
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J (2017) Crop production under drought and heat stress: Plant responses and management options. Front Plant Sci 8:1–16. https://doi.org/10.3389/fpls.2017.01147
FAO and WHO (2019) Codex Alimentarius Commission – procedural manual twenty-seventh edition. Rome, p 254
FAOSTAT (2020) FAOSTAT [WWW Document]. Food Agric. Organ. United Nations. URL https://www.fao.org/faostat/en/#data/QCL (accessed 7.30.22)
Fernandes VC, Lehotay SJ, Geis-Asteggiante L, Kwon H, Mol HGJ, van der Kamp H, Mateus N, Domingues VF, Delerue-Matos C (2014) Analysis of pesticide residues in strawberries and soils by GC-MS/MS, LC-MS/MS and two-dimensional GC-time-of-flight MS comparing organic and integrated pest management farming 31: 262-270.https://doi.org/10.1080/19440049.2013.865842
Han Y, Xu J, Dong F, Li W, Liu X, Li Y, Kong Z, Zhu Y, Liu N, Zheng Y (2013) The fate of spirotetramat and its metabolite spirotetramat-enol in apple samples during apple cider processing. Food Control 34:283–290. https://doi.org/10.1016/J.FOODCONT.2013.05.009
Handford CE, Elliott CT, Campbell K (2015) A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr Environ Assess Manag 11:525–536. https://doi.org/10.1002/ieam.1635
Hong JH, Lee CR, Lim JS, Lee KS (2011) Comparison of Analytical Methods and Residue Patterns of Pymetrozine in Aster scaber. Bull Environ Contam Toxicol 87:649. https://doi.org/10.1007/S00128-011-0407-8
Hoskins WM (1961) Mathematical Treatment of the Rate of Loss of Pesticide Residue. FAO Plant Prot Bull 9:163–168
Jang J, Rahman MM, Ko AY, Abd El-Aty AM, Park JH, Cho SK, Shim JH (2014) A matrix sensitive gas chromatography method for the analysis of pymetrozine in red pepper: Application to dissipation pattern and PHRL. Food Chem 146:448–454. https://doi.org/10.1016/J.FOODCHEM.2013.09.052
Jia G, Zeng L, Zhao S, Ge S, Long X, Zhang Y, Hu D (2019) Monitoring residue levels and dietary risk assessment of pymetrozine for Chinese consumption of cauliflower. Biomed Chromatogr 33:e4455. https://doi.org/10.1002/BMC.4455
Kay IR, Herron GA (2010) Evaluation of existing and new insecticides including spirotetramat and pyridalyl to control Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) on peppers in Queensland. Aust J Entomol 49:175–181. https://doi.org/10.1111/J.1440-6055.2010.00751.X
Kumar BV, Kuttalam S (2009) Toxicity of spirotetramat 150 OD - a new insecticide molecule against natural enemies of chillies. J Plant Prot Environ 6:1–5
Kumar BV, Kuttalam S, Chandrasekaran S (2009) Efficacy of a new insecticide spirotetramat against cotton whitefly. Pestic Res J 21:45–48
Lashkari MR, Sahragard A, Ghadamyari M (2007) Sublethal effects of imidacloprid and pymetrozine on population growth parameters of cabbage aphid, Brevicoryne brassicae on rapeseed, Brassica napus L. Insect Sci 14:207–212. https://doi.org/10.1111/J.1744-7917.2007.00145.X
Lehotay SJ (2007) Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: collaborative study. J AOAC Int. 90:485–520
Lehotay SJ, De Kok A, Hiemstra M, Van Bodegraven P (2005) Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J AOAC Int 88. https://doi.org/10.1093/jaoac/88.2.595
Li C, Yang T, Huangfu W, Wu Y (2011) Residues and dynamics of pymetrozine in rice field ecosystem. Chemosphere 82:901–904. https://doi.org/10.1016/J.CHEMOSPHERE.2010.10.053
Li S, Liu X, Dong F, Xu J, Xu H, Hu M, Zheng Y (2016) Chemometric-assisted QuEChERS extraction method for the residual analysis of thiacloprid, spirotetramat and spirotetramat’s four metabolites in pepper: Application of their dissipation patterns. Food Chem 192:893–899. https://doi.org/10.1016/J.FOODCHEM.2015.07.122
Liang H, Li L, Li W, Wu Y, Zhou Z, Liu F (2011) Dissipation and residue of dimethomorph in pepper and soil under field conditions. Ecotoxicol Environ Saf 74:1331–1335. https://doi.org/10.1016/J.ECOENV.2011.02.009
MacLachlan DJ, Hamilton D (2010) Estimation methods for Maximum Residue Limits for pesticides. Regul Toxicol Pharmacol 58:208–218. https://doi.org/10.1016/J.YRTPH.2010.05.012
Malhat F, Abdallah O (2019) Residue distribution and risk assessment of two macrocyclic lactone insecticides in green onion using micro-liquid-liquid extraction (MLLE) technique coupled with liquid chromatography tandem mass spectrometry. Environ Monit Assess 191:584. https://doi.org/10.1007/s10661-019-7752-1
Mohapatra S, Deepa M, Jagadish GK (2012a) An Efficient Analytical Method for Analysis of Spirotetramat and its Metabolite Spirotetramat-Enol by HPLC. Bull Environ Contam Toxicol 88:124–128. https://doi.org/10.1007/s00128-011-0494-6
Mohapatra S, Deepa M, Lekha S, Nethravathi B, Radhika B, Gourishanker S (2012b) Residue Dynamics of Spirotetramat and Imidacloprid in/on Mango and Soil. Bull Environ Contam Toxicol 89:862–867. https://doi.org/10.1007/s00128-012-0762-0
Mohapatra S, Kumar S, Prakash GS (2015) Residue evaluation of imidacloprid, spirotetramat, and spirotetramat-enol in/on grapes (Vitis vinifera L.) and soil. Environ Monit Assess 187:1–12. https://doi.org/10.1007/S10661-015-4859-X/FIGURES/4
Pandiselvi S, Sathiyanarayanan S, Ramesh A (2010) Determination of Spirotetramat and Imidacloprid Residues in Cotton Seed, Lint, Oil and Soil by HPLC UV Method and their Dissipation in Cotton Plant. Pestic Res J 22:168–173
Saber AN, Farag M, Anagnostopoulos C, Kasiotis KM (2020) Evaluation of dissipation, unit-unit-variability and terminal residue of etoxazole residues in strawberries from two different parts in Egypt. J Consum Prot Food Saf 15:229–236. https://doi.org/10.1007/s00003-019-01266-w
SANTE (2021) Guidance Document on Pesticide Analytical Methods for Risk Assessment and Post-approval Control and Monitoring Purposes. Sante/2020/12830 1–50
Shen G, Hu X, Hu Y (2009) Kinetic study of the degradation of the insecticide pymetrozine in a vegetable-field ecosystem. J Hazard Mater 164:497–501. https://doi.org/10.1016/J.JHAZMAT.2008.08.020
Singh B, Mandal K, Sahoo SK, Bhardwaj U, Battu RS (2013) Development and Validation of an HPLC Method for Determination of Spirotetramat and Spirotetramat cis enol in Various Vegetables and Soil. J AOAC Int 96:670–675. https://doi.org/10.5740/jaoacint.11-185
Singh B, Mandal K, Sahoo SK, Bhardwaj U, Battu RS (2013) Development and validation of an HPLC method for determination of spirotetramat and spirotetramat cis enol in various vegetables and soil. J. AOAC Int 96:670–675. https://doi.org/10.5740/jaoacint.11-185
Smiley RW, Marshall JM, Yan GP (2011) Effect of Foliarly Applied Spirotetramat on Reproduction of Heterodera avenae on Wheat Roots 95: 983–989.https://doi.org/10.1094/PDIS-01-11-0017
Taylor PJ (2005) Matrix effects: The Achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry. Clin Biochem. https://doi.org/10.1016/j.clinbiochem.2004.11.007
Vemuri S, Rao CS, Swarupa S (2014) Dissipation of spirotetramat and imidacloprid in grapes and soil. J Multidiscip Eng Sci Technol 1:319–324
Xu F, Ren W, Fang X, Chen L, Zha X (2021) Residues, dissipation, and safety evaluation of pymetrozine-clothianidin mixture in strawberry. Environ Sci Pollut Res 28:22641–22650. https://doi.org/10.1007/s11356-020-12223-8
Xun Q, Zhenshan Z, Yongda C, Shaojun Z, Junfeng G, Lixin F, Xudong Z, Mengyuan Q (2019) Residues and dissipation dynamics of spirotetramat and its metabolites in pear and soil QIAN. Chinese J Pestic Sci 21:338–344
Yang Y, Kong W, Zhao L, Xiao Q, Liu H, Zhao X, Yang M (2015) A multiresidue method for simultaneous determination of 44 organophosphorous pesticides in Pogostemon cablin and related products using modified QuEChERS sample preparation procedure and GC–FPD. J Chromatogr B 974:118–125. https://doi.org/10.1016/J.JCHROMB.2014.10.023
Zhang X, Cheng X, Wang C, Xi Z, Li Q (2007) Efficient High-Performance Liquid Chromatography with Liquid-Liquid Partition Cleanup Method for the Determination of Pymetrozine in Tobacco. Ann Chim 97:295–301. https://doi.org/10.1002/adic.200790015
Zhang Y, Zhang L, Xu P, Li J, Wang H (2015) Dissipation and residue of pymetrozine in rice field ecosystem. Environ Monit Assess 187:78. https://doi.org/10.1007/s10661-014-4256-x
Zhu Y, Liu X, Xu J, Dong F, Liang X, Li M, Duan L, Zheng Y (2013) Simultaneous determination of spirotetramat and its four metabolites in fruits and vegetables using a modified quick, easy, cheap, effective, rugged, and safe method and liquid chromatography/tandem mass spectrometry. J Chromatogr A 1299:71–77. https://doi.org/10.1016/J.CHROMA.2013.05.049
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Farag Malhat, Osama Abdallah and Mona Bakery contributed in writing the first draft of the manuscript. Mona Bakery performed the collection of real samples, Mona Bakery, Farag Malhat, and Osama Abdallah performed samples chemical analysis. Walaa Abd El Ghany, Amira Abdallah, Mohamed Tawfic, Sarah Griesh, Mona Gaber, Indra Purnama and Shokr Abdelsalam performed data analysis and revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors have participated in the process, read and agreed to the published version of the manuscript.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Malhat, F., Bakery, M., Abdallah, O. et al. Dissipation kinetics and exposure of spirotetramat and pymetrozine in open fields, a prelude to risk assessment of green bean consumption. Environ Sci Pollut Res 30, 57747–57758 (2023). https://doi.org/10.1007/s11356-023-26100-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26100-7